By
Many Substances Used For Food Processing Are Never Listed On Ingredient Labels
https://ift.tt/JQDl1wU
Authored by Flora Zhao via The Epoch Times (emphasis ours),
The long list of unfamiliar names on ingredient labels of processed foods is already a cause for concern. However, many people are unaware of another category of additives never listed on these labels. These “invisible” additives are known as processing aids.

Processing aids serve various roles in food production. They can soak and wash ingredients, filter beverages like wine or juice to make them clearer, or improve the texture of bread to make it softer and more elastic. During the production process, these aids are consumed, transformed, or removed, rendering them virtually undetectable in the final product.
Take fruit juice as an example. Using enzymes for juice extraction is a common production method, which can result in juice yields exceeding 90 percent of the fruit’s weight. By treating the raw fruit materials with several enzymes at a specific temperature for a few hours, the fruit “liquefies.” Specifically, cellulase breaks down the cell walls of the fruit, releasing more juice and sugars, while pectinase and amylase break down polysaccharides such as pectin. These enzymes improve the flow of the juice in processing containers and enhance its sweetness. They are consumed and transformed during processing, ultimately not appearing on the ingredient label.
Another example is regular milk supplemented with lactase, which becomes low-lactose milk, whereas adding rennet turns it into cheese. Additionally, applying palm wax to baking molds aids in easy cake release. Bottled sauces often have nitrogen added during bottling to displace oxygen, preventing oxidation and product spoilage.
Processing aids include a variety of substances used in food production, including clarifying agents, clouding agents, catalysts, flocculants, filter aids, and crystallization inhibitors. These aids perform essential functions such as improving texture, enhancing clarity, and preventing spoilage.
Some people might worry, as these substances are not stated on the label, Martin Bucknavage, a senior food safety specialist at Penn State University’s Department of Food Science, told The Epoch Times. However, he said that there is no need for excessive concern.
“There [are] risks in all processes; there [are] definitely potential side effects and negative aspects that need to be looked into,” Tim Bowser, the food process engineer at the Robert M. Kerr Food and Agricultural Products Center at Oklahoma State University, told The Epoch Times. Yet unlike additives, the nature of processing aids determines that “they do not have that ability to cheat” and are less likely to be used for deception and adulteration.
In real-life scenarios, “the residuals would be too low to detect,” Mr. Bowser said.
However, he noted that with the continuous development of detection technologies, some companies are now capable of detecting substances at levels as low as parts per billion or even trillion. Furthermore, the safety of processing aids is constantly being evaluated, and as people’s understanding grows, regulations governing their use may be adjusted, or “something could be removed from the generally regarded as safe list.”
Alcohol, Juice, and Heavy Metals
Despite Germany’s stringent centuries-old laws governing beer production methods, routine analyses have uncovered a gradual increase in arsenic content in German beer, with diatomaceous earth being considered a potential source.
Diatomaceous earth is commonly used to filter alcohol and beverages to increase their yield.
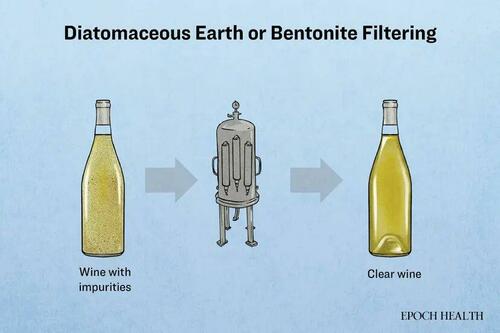
To test this hypothesis, researchers mixed diatomaceous earth with beer and analyzed the filtrate for trace metals, finding elevated levels of arsenic and aluminum.
Diatomaceous earth is a fossilized sedimentary deposit formed from the cell walls of ancient diatoms that settled on the ocean floor. After extraction, it is ground into a powder and primarily composed of silicon dioxide.
Another often-used beverage filter during the manufacturing process is bentonite, a clay with adsorption capabilities, which the FDA classifies as “generally recognized as safe.”
Since diatomaceous earth and bentonite are derived from mined materials, “they can contain a large array of elements, including heavy metals,” wrote Benjamin W. Redan, a U.S. Food and Drug Administration (FDA) scientist, in a 2020 study published in the Journal of Agricultural and Food Chemistry.
A study conducted by the FDA and the University of Maryland researchers indicated that diatomaceous earth can increase arsenic levels in apple juice by more than five times, while arsenic levels in grape juice increased by 67 percent.
Additionally, researchers have discovered that adding bentonite can increase vanadium levels in apple juice from around 3 μg/kg to up to 200 μg/kg. While this does not reach toxic levels, the increase is notable.
The quality of different processing aids varies. In January 2023, Hungarian researchers published a study in the journal Foods. They added 21 types of commercial bentonite products to white wine and found that while some showed no significant change in lead content, others increased considerably. For instance, one type of bentonite increased the lead content from 2.27 µg/L to 9.46 µg/L, marking a rise of over 300 percent.
“The use of certain processing aids can increase levels of contaminants in beverages,” the FDA spokesperson told The Epoch Times. “The FDA has issued draft guidance that notes that changing or treating filter aids may reduce contaminants released during filtration,” he added.
Hidden Concerns of Decaffeinated Coffee
A processing aid called methylene chloride is employed to remove caffeine from coffee beans, producing decaffeinated coffee.
Methylene chloride is a highly efficient solvent but is often considered hazardous. In the liver, it metabolizes to produce significant amounts of carbon monoxide and formaldehyde, the latter being a known carcinogen. In animal models, methylene chloride has shown hepatotoxicity, neurotoxicity, and potential carcinogenic effects.
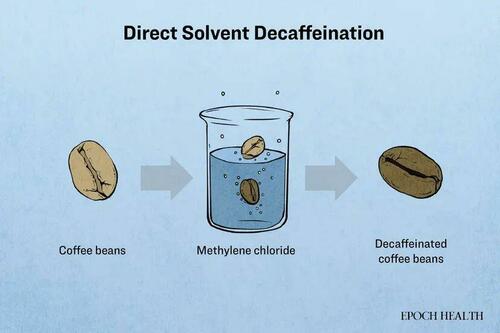
The FDA regulations specify that the residue level of methylene chloride in food must not exceed 10 parts per million (ppm), which is equivalent to 10 mg/kg or 10,000 μg/kg.
While methylene chloride’s high volatility generally facilitates the removal of its residues, they can still persist, and residues in some products may be relatively high.
Considering that decaffeinated coffee is a preferred choice for sensitive groups such as pregnant women, people with cardiovascular diseases, and those with neurological conditions, some people have raised concerns about the use of methylene chloride in its production.
The nonprofit organization Clean Label Project, which has long focused on the coffee industry’s use of methylene chloride in producing decaffeinated coffee, commissioned a professional analytical company in 2022 to conduct a double-blind test on 17 decaffeinated products. The results showed that although all products had methylene chloride levels below the FDA’s set standards, one product contained 8,931 μg/kg, close to the upper limit, while two other products had residue levels between 3,500 and 4,000 μg/kg.
“Anything that’s used like that, that is known to be [a problem] should be continuously looked at,” Mr. Bowser said when discussing the use of methylene chloride in the production of decaffeinated coffee. He emphasized that if a substance is deemed hazardous, it remains dangerous, regardless of the residual amount.
Mr. Bowser also underscored the importance of ongoing scrutiny and openness to diverse perspectives regarding certain widely used substances currently considered safe, such as hexane used in soybean oil extraction.
Hexane and Vegetable Oil
Traditional mechanical pressing methods for extracting vegetable oil typically achieve extraction rates ranging from 60 to 80 percent for oilseed crops. In contrast, chemical solvent extraction, which is now predominantly used, can achieve rates close to 100 percent.
Hexane, a commonly used solvent in this process, is a hydrocarbon extracted from crude oil. It remains liquid at room temperature but is highly volatile.
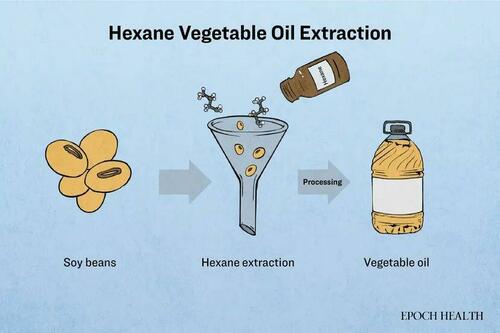
In the extraction of vegetable oils such as canola, sunflower, and cottonseed, the oilseeds undergo cleaning, crushing, steaming, and drying before being immersed in hexane. Following the principle of “like dissolves like,” lipids from the seeds are released, while hexane is subsequently evaporated using hot steam. The extracted oil then undergoes further refining processes, while hexane is collected and reused.
Hexane is also employed in the extraction of flavors, color additives, and other bioactive ingredients in addition to vegetable oils.
Numerous studies have identified hexane as neurotoxic to humans. According to the U.S. Environmental Protection Agency (EPA), short-term exposure to hexane can cause irritation, headaches, and dizziness, while prolonged exposure can lead to nerve damage.
The EPA has set a reference dose (RfD) for hexane exposure based on animal toxicity studies, establishing a daily limit of 0.06 mg/kg/day for humans. For a person weighing 70 kilograms, this provisional reference dose equates to a maximum of 4.2 milligrams per day. The European Medicines Agency classifies hexane as a Class 2 solvent, meaning it should be limited, and has established a “Permitted Daily Exposure” similar to that of the EPA.
Different countries have varying regulations regarding hexane residues in edible oils. For example, the European Union’s standard is 1 mg/kg. Some vegetable oils in developing countries have been found to exceed the EU’s hexane residue limit. From an environmental perspective, although most hexane is recovered during the production process, some is still released into the air and can enter the food chain. Recent estimates indicate that an additional one million tons of hexane are needed globally each year to compensate for losses during the extraction process.
The FDA currently has no regulations regarding hexane residue levels in edible oil products. “To ensure that vegetable oil is sufficiently purified to minimize levels of contaminants like hexane, manufacturers may set a limit that only allows for trace amounts of hexane in the final product.” The FDA spokesperson told The Epoch Times, “The FDA does not typically sample vegetable oils for residual hexane ... based on the information that we have, any residual levels would be very low, if detectable if added to food.”
Driven by concerns about hexane as an extraction solvent, some processors are shifting towards healthier extraction methods. These methods include aqueous-assisted enzyme extraction, natural solvent extraction (such as from citrus peel and tree oils), and more advanced mechanical pressing methods with higher oil yields.
Enzymes in Bread: A Seemingly Harmless Aid
There is another major category of processing aids: enzymes, widely utilized in baking products such as bread.
Xylanases have been employed in baking for several decades. They degrade polysaccharides in flour, resulting in fluffier bread. Proteases break down large protein molecules in the dough into smaller ones, making the dough softer and more malleable. They also expedite dough fermentation, enhancing the texture and flavor of bread. Additionally, by breaking down more proteins into amino acids, proteases enrich the nutritional value of bread and facilitate absorption. Alpha-amylase (α-amylase) breaks down starch in the dough into sugars, improving the softness, elasticity, and sweetness of the bread. Additionally, it reduces moisture content in bread and regulates microbial growth, thereby extending its shelf life.
Read more here...
Loading...
news
via Government Slaves https://ift.tt/T2qgPW9
July 16, 2024 at 06:08AM
July 16, 2024 at 07:31AM
via ZeroHedge News https://ift.tt/JQDl1wU
