Authored by Sina McCullough via The Epoch Times (emphasis ours),
As spring gardening approaches, a new contender has entered the fray—the genetically modified (GM) Purple Tomato. Unlike its GM predecessors, the GM Purple Tomato is not destined solely for the fields of commercial agriculture—it has made its debut in the backyards of home gardeners across the United States.
With claims of heightened antioxidant levels and potential health benefits, this novel creation has stirred both excitement and controversy among consumers and scientists alike. Biotech investors hope it can usher in a new era of public trust in genetically engineered foods while skeptics worry the tomatoes’ near-total lack of regulation or review may hide dangers to human health and/or the environment.
Development
The GM Purple Tomato was engineered by scientists at Norfolk Plant Sciences in the UK. Led by biochemist Cathie Martin and her team, the project aimed to harness the natural properties of anthocyanins, compounds found in blueberries and blackberries, to enhance the nutritional profile of tomatoes.

Using genetic engineering techniques, Martin and her colleagues inserted two genes responsible for purple coloration in edible snapdragon flowers into tomato plants. This process enabled the tomatoes to express the genes from the snapdragon and, subsequently, produce high levels of anthocyanins, thereby imbuing the tomatoes with a distinct purple hue and potentially enhanced health benefits.
According to Norfolk Healthy Produce, the U.S. subsidiary of Norfolk Plant Sciences, the Purple Tomatoes are a “rich source of antioxidants” due to the increased content of anthocyanins. Unlike domesticated tomatoes which contain anthocyanins in the skin, the Purple Tomato contains anthocyanins throughout the whole tomato.
The genesis of the GM Purple Tomato marks a significant milestone in agricultural biotechnology. Unlike previous GM crops primarily targeted at commercial producers, this tomato is the first GM food crop directly marketed to home gardeners in the United States, offering an opportunity for individuals to engage with biotechnology in their own backyard.
According to Norfolk Healthy Produce, more than 13,000 Purple Tomato seed orders have already shipped.
Regulatory Approval
The GM Purple Tomato was deregulated by the U.S. Department of Agriculture (USDA) in 2022. According to a statement from the USDA, the GM Purple Tomato is not subject to regulation by the USDA because it does not pose a plant pest risk:
“With respect to Norfolk Plant Sciences’ purple tomato, we did not identify any plausible pathways to increased plant pest risk compared to other cultivated tomatoes and issued a response letter indicating the plant is not subject to regulation.”
In 2023, the Purple Tomato received a “no questions” letter from the U.S. Food and Drug Administration (FDA), which means the Purple Tomato is considered “generally recognized as safe” (GRAS) and, therefore, does not require premarket review or approval by the FDA.
To qualify for GRAS status, Norfolk Plant Sciences submitted data from tests conducted internally.

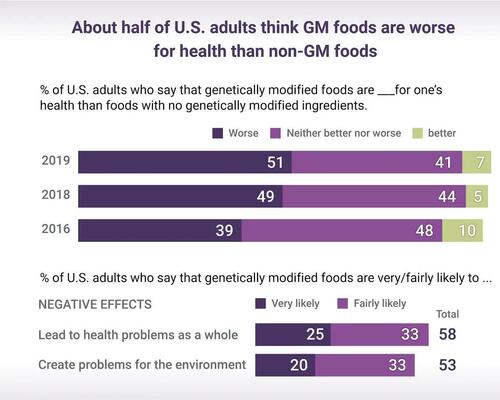
The lack of safety testing by the USDA and FDA, as well as reliance on data generated by the company that will profit from approval of its own product, has led to some experts calling for a more comprehensive safety assessment.
Safety Concerns and Health Claims
Data provided to the FDA by Norfolk Plant Sciences demonstrates the company conducted various safety tests. However, critics argue the tests are insufficient to guarantee the safety of the Purple Tomato for human consumption.
According to an FDA memo dated June 13, 2023, tests conducted by Norfolk Plant Sciences mainly focused on six areas. Of those, four were relatively straightforward while two have raised safety concerns among experts, according to GM Watch.
1. PCR and Southern blot analysis were conducted by Norfolk Plant Sciences to determine if the snapdragon foreign DNA was inserted into the tomato DNA.
- The company (Norfolk Plant Sciences) stated that insertion of the foreign DNA was confirmed.
2. PCR and sequence comparison of DNA samples were conducted to confirm the stability of the inheritability of the insertion across generations. Plants were bred to determine if the purple phenotype was inherited in a Mendelian segregation fashion.
- The company stated the purple phenotype was inheritable.
3. Compositional analysis was conducted to determine if the Purple Tomato contained similar nutrients at similar levels compared with non-GMO tomatoes, including protein, fat, carbohydrate, fiber, minerals, carotenoids, vitamins, and alpha-tomatine.
- The company determined the levels of most of the nutritional components to be similar or with “minor differences.”
4. Norfolk Plant Sciences assessed dietary exposure levels assuming the complete replacement of red tomatoes in the human diet with the Purple Tomato for two days.
- The company concluded the level of dietary exposure to anthocyanins is the same as consuming high-anthocyanin foods. For example, 8 ounces of Purple Tomato juice is equivalent to consuming 1 cup of blueberries.
The Controversial Tests
1. Bioinformatic analyses were utilized to determine if any open reading frames were generated or disrupted by inserting the foreign DNA. Norfolk Plant Sciences searched the DNA sequences flanking the insertion sequence in the tomatoes.
- The company reported no open reading frames flanking the insertion location.
Since Norfolk Plant Sciences did not assess possible damage to the entire genome using advanced laboratory techniques, geneticist Michael Antoniou expressed concern in a statement published by GM Watch.
“There’s no evidence that the developers of the GM purple tomato have carried out the kind of molecular analyses (proteomics and metabolomics) that could help establish whether they only got the change they want, with no unintended changes. As a result, we don’t know if these tomatoes are safe to eat,” said Mr. Antoniou.
“We must also bear in mind that the GM transformation process (plant tissue culture and plant cells transformation) will inevitably give rise to hundreds if not thousands of sites of unintended DNA damage (mutations). These wide scale mutations can change patterns of gene function and alter biochemistry and composition, with unknown downstream health consequences,” he said.
2. Assessment of new peptides of equal or greater than 30 amino acids at the insertion site of the foreign DNA was conducted to rule out toxicity or allergenicity concerns.
- The company identified one “putative” peptide, however, they stated, “this peptide has no homology to any known allergen or protein and there was no evidence this sequence is transcribed in tomato.” They concluded the results “do not raise food safety concerns.”
Allergenicity is an ongoing concern regarding the genetic modification of food. For example, a study published in Nature in 1999 reported that bean plants were genetically modified to produce higher levels of methionine and cysteine but were discarded because the expressed protein of the transgene was highly allergenic.
While Norfolk Plant Sciences did not identify a match with any known allergens, that does not guarantee the peptide formed through the process of gene modification is not an allergen. Given that nearly 11 percent of adults and 5.6 million children in the United States have food allergies, it may be prudent to apply the precautionary principle when modifying our food’s genetic makeup.
The Test That Everyone Talked About
Although not included in the 2023 FDA memo, Norfolk Plant Sciences, in conjunction with Cathie Martin, published a pilot feeding study in 2008 in Nature Biotechnology that examined the effects of Purple Tomato supplementation on the life span of cancer-susceptible mice.
According to the study, mice fed the GM tomato lived longer—by an average of 40 days than those fed non-GM red tomatoes.
Publication of the pilot study prompted the John Innes Centre to publish a press release titled, “Purple tomatoes may keep cancer at bay.” (Norfolk Plant Sciences is a spinoff company from the John Innes Centre.)
Read more here...
Authored by Sina McCullough via The Epoch Times (emphasis ours),
As spring gardening approaches, a new contender has entered the fray—the genetically modified (GM) Purple Tomato. Unlike its GM predecessors, the GM Purple Tomato is not destined solely for the fields of commercial agriculture—it has made its debut in the backyards of home gardeners across the United States.
With claims of heightened antioxidant levels and potential health benefits, this novel creation has stirred both excitement and controversy among consumers and scientists alike. Biotech investors hope it can usher in a new era of public trust in genetically engineered foods while skeptics worry the tomatoes’ near-total lack of regulation or review may hide dangers to human health and/or the environment.
Development
The GM Purple Tomato was engineered by scientists at Norfolk Plant Sciences in the UK. Led by biochemist Cathie Martin and her team, the project aimed to harness the natural properties of anthocyanins, compounds found in blueberries and blackberries, to enhance the nutritional profile of tomatoes.

Using genetic engineering techniques, Martin and her colleagues inserted two genes responsible for purple coloration in edible snapdragon flowers into tomato plants. This process enabled the tomatoes to express the genes from the snapdragon and, subsequently, produce high levels of anthocyanins, thereby imbuing the tomatoes with a distinct purple hue and potentially enhanced health benefits.
According to Norfolk Healthy Produce, the U.S. subsidiary of Norfolk Plant Sciences, the Purple Tomatoes are a “rich source of antioxidants” due to the increased content of anthocyanins. Unlike domesticated tomatoes which contain anthocyanins in the skin, the Purple Tomato contains anthocyanins throughout the whole tomato.
The genesis of the GM Purple Tomato marks a significant milestone in agricultural biotechnology. Unlike previous GM crops primarily targeted at commercial producers, this tomato is the first GM food crop directly marketed to home gardeners in the United States, offering an opportunity for individuals to engage with biotechnology in their own backyard.
According to Norfolk Healthy Produce, more than 13,000 Purple Tomato seed orders have already shipped.
Regulatory Approval
The GM Purple Tomato was deregulated by the U.S. Department of Agriculture (USDA) in 2022. According to a statement from the USDA, the GM Purple Tomato is not subject to regulation by the USDA because it does not pose a plant pest risk:
“With respect to Norfolk Plant Sciences’ purple tomato, we did not identify any plausible pathways to increased plant pest risk compared to other cultivated tomatoes and issued a response letter indicating the plant is not subject to regulation.”
In 2023, the Purple Tomato received a “no questions” letter from the U.S. Food and Drug Administration (FDA), which means the Purple Tomato is considered “generally recognized as safe” (GRAS) and, therefore, does not require premarket review or approval by the FDA.
To qualify for GRAS status, Norfolk Plant Sciences submitted data from tests conducted internally.

The lack of safety testing by the USDA and FDA, as well as reliance on data generated by the company that will profit from approval of its own product, has led to some experts calling for a more comprehensive safety assessment.
Safety Concerns and Health Claims
Data provided to the FDA by Norfolk Plant Sciences demonstrates the company conducted various safety tests. However, critics argue the tests are insufficient to guarantee the safety of the Purple Tomato for human consumption.
According to an FDA memo dated June 13, 2023, tests conducted by Norfolk Plant Sciences mainly focused on six areas. Of those, four were relatively straightforward while two have raised safety concerns among experts, according to GM Watch.
1. PCR and Southern blot analysis were conducted by Norfolk Plant Sciences to determine if the snapdragon foreign DNA was inserted into the tomato DNA.
- The company (Norfolk Plant Sciences) stated that insertion of the foreign DNA was confirmed.
2. PCR and sequence comparison of DNA samples were conducted to confirm the stability of the inheritability of the insertion across generations. Plants were bred to determine if the purple phenotype was inherited in a Mendelian segregation fashion.
- The company stated the purple phenotype was inheritable.
3. Compositional analysis was conducted to determine if the Purple Tomato contained similar nutrients at similar levels compared with non-GMO tomatoes, including protein, fat, carbohydrate, fiber, minerals, carotenoids, vitamins, and alpha-tomatine.
- The company determined the levels of most of the nutritional components to be similar or with “minor differences.”

4. Norfolk Plant Sciences assessed dietary exposure levels assuming the complete replacement of red tomatoes in the human diet with the Purple Tomato for two days.
- The company concluded the level of dietary exposure to anthocyanins is the same as consuming high-anthocyanin foods. For example, 8 ounces of Purple Tomato juice is equivalent to consuming 1 cup of blueberries.
The Controversial Tests
1. Bioinformatic analyses were utilized to determine if any open reading frames were generated or disrupted by inserting the foreign DNA. Norfolk Plant Sciences searched the DNA sequences flanking the insertion sequence in the tomatoes.
- The company reported no open reading frames flanking the insertion location.
Since Norfolk Plant Sciences did not assess possible damage to the entire genome using advanced laboratory techniques, geneticist Michael Antoniou expressed concern in a statement published by GM Watch.
“There’s no evidence that the developers of the GM purple tomato have carried out the kind of molecular analyses (proteomics and metabolomics) that could help establish whether they only got the change they want, with no unintended changes. As a result, we don’t know if these tomatoes are safe to eat,” said Mr. Antoniou.
“We must also bear in mind that the GM transformation process (plant tissue culture and plant cells transformation) will inevitably give rise to hundreds if not thousands of sites of unintended DNA damage (mutations). These wide scale mutations can change patterns of gene function and alter biochemistry and composition, with unknown downstream health consequences,” he said.
2. Assessment of new peptides of equal or greater than 30 amino acids at the insertion site of the foreign DNA was conducted to rule out toxicity or allergenicity concerns.
- The company identified one “putative” peptide, however, they stated, “this peptide has no homology to any known allergen or protein and there was no evidence this sequence is transcribed in tomato.” They concluded the results “do not raise food safety concerns.”
Allergenicity is an ongoing concern regarding the genetic modification of food. For example, a study published in Nature in 1999 reported that bean plants were genetically modified to produce higher levels of methionine and cysteine but were discarded because the expressed protein of the transgene was highly allergenic.
While Norfolk Plant Sciences did not identify a match with any known allergens, that does not guarantee the peptide formed through the process of gene modification is not an allergen. Given that nearly 11 percent of adults and 5.6 million children in the United States have food allergies, it may be prudent to apply the precautionary principle when modifying our food’s genetic makeup.
The Test That Everyone Talked About
Although not included in the 2023 FDA memo, Norfolk Plant Sciences, in conjunction with Cathie Martin, published a pilot feeding study in 2008 in Nature Biotechnology that examined the effects of Purple Tomato supplementation on the life span of cancer-susceptible mice.
According to the study, mice fed the GM tomato lived longer—by an average of 40 days than those fed non-GM red tomatoes.
Publication of the pilot study prompted the John Innes Centre to publish a press release titled, “Purple tomatoes may keep cancer at bay.” (Norfolk Plant Sciences is a spinoff company from the John Innes Centre.)
Read more here...